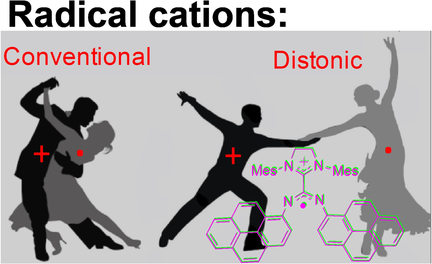
An N-Heterocyclic-Carbene-Derived Distonic Radical Cation
Nolan M. Gallagher
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorHong-Zhou Ye
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorShuting Feng
Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Research Laboratory of Electronics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorJeffrey Lopez
Research Laboratory of Electronics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Departments of Mechanical Engineering and Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorYun Guang Zhu
Research Laboratory of Electronics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Departments of Mechanical Engineering and Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorTroy Van Voorhis
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorYang Shao-Horn
Research Laboratory of Electronics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Departments of Mechanical Engineering and Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorCorresponding Author
Jeremiah A. Johnson
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorNolan M. Gallagher
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorHong-Zhou Ye
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorShuting Feng
Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Research Laboratory of Electronics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorJeffrey Lopez
Research Laboratory of Electronics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Departments of Mechanical Engineering and Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorYun Guang Zhu
Research Laboratory of Electronics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Departments of Mechanical Engineering and Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorTroy Van Voorhis
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorYang Shao-Horn
Research Laboratory of Electronics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Departments of Mechanical Engineering and Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorCorresponding Author
Jeremiah A. Johnson
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, 02139 USA
Search for more papers by this authorGraphical Abstract
A persistent distonic radical cation was prepared through one-electron oxidation of an N-heterocyclic carbene–carbodiimide (NHC–CDI) adduct bearing pyrene substituents. Application of this compound in an organic redox-flow battery was demonstrated, setting the stage for the development of novel redox-active NHC–CDIs for various applications.
Abstract
We present the discovery of a novel radical cation formed through one-electron oxidation of an N-heterocyclic carbene–carbodiimide (NHC–CDI) zwitterionic adduct. This compound possesses a distonic electronic structure (spatially separate spin and charge regions) and displays persistence under ambient conditions. We demonstrate its application in a redox-flow battery exhibiting minimal voltage hysteresis, a flat voltage plateau, high Coulombic efficiency, and no performance decay for at least 100 cycles. The chemical tunability of NHCs and CDIs suggests that this approach could provide a general entry to redox-active NHC–CDI adducts and their persistent radical ions for various applications.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201915534-sup-0001-misc_information.pdf2.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. R. Benson, E. M. Fatila, S. Lee, M. G. Marzo, M. Pink, M. B. Mills, K. E. Preuss, A. H. Flood, J. Am. Chem. Soc. 2016, 138, 15057–11506.
- 2G. Ulas, T. Lemmin, Y. Wu, G. T. Gassner, W. F. Degrado, Nat. Chem. 2016, 8, 354–359.
- 3F. Dénès, M. Pichowicz, G. Povie, P. Renaud, Chem. Rev. 2014, 114, 2587–2693.
- 4K. J. Romero, M. S. Galliher, D. A. Pratt, C. R. J. Stephenson, Chem. Soc. Rev. 2018, 47, 7851–7866.
- 5V. Sciannamea, R. Jérôme, C. Detembleur, Chem. Rev. 2008, 108, 1104–1126.
- 6K. A. Payne, P. Nesvadba, J. Debling, M. F. Cunningham, R. A. Hutchinson, ACS Macro Lett. 2015, 4, 280–283.
- 7Y. Guillaneuf, D. Bertin, D. Gigmes, D.-L. Versace, J. Lalevée, J.-P. Fouassier, Macromolecules 2010, 43, 2204–2212.
- 8T. B. Schon, B. T. McAllister, P.-F. Li, D. S. Seferos, Chem. Soc. Rev. 2016, 45, 6345–6404.
- 9J. Friedl, M. A. Lebedeva, K. Porfyrakis, U. Stimming, T. W. Chamberlain, J. Am. Chem. Soc. 2018, 140, 401–405.
- 10Y. Yan, S. G. Robinson, M. S. Sigman, M. S. Sanford, J. Am. Chem. Soc. 2019, 141, 15301–15306.
- 11G. Gil Alvarajedo, H.-V. T. Nguyen, P. Harvey, N. M. Gallagher, D. Le, M. F. Ottaviani, A. Jasanoff, G. Delaittre, J. A. Johnson, ACS Macro Lett. 2019, 8, 473–478.
- 12H.-V. T. Nguyen, A. Detappe, N. M. Gallagher, H. Zhang, P. Harvey, C. Yan, C. Mathieu, M. R. Golder, Y. Jiang, M. F. Ottaviani, A. Jasanoff, A. Rajca, I. M. Ghobrial, P. P. Ghorogchian, J. A. Johnson, ACS Nano 2018, 12, 11343–11354.
- 13H.-V. T. Nguyen, Q. Chen, J. T. Paletta, P. Harvey, Y. Jiang, H. Zhang, M. D. Boska, M. F. Ottaviani, A. Jasanoff, A. Rajca, J. A. Johnson, ACS Cent. Sci. 2017, 3, 820–822.
- 14M. F. Silva Valverde, P. Scweyen, D. Gisinger, T. Bannenberg, M. Freytag, C. Kleeberg, M. Tamm, Angew. Chem. Int. Ed. 2017, 56, 1135–1140;
Angew. Chem. 2017, 129, 1155–1160.
10.1002/ange.201610903 Google Scholar
- 15J. Back, J. Park, Y. Kim, H. Kang, Y. Kim, M. J. Park, K. Kim, E. Lee, J. Am. Chem. Soc. 2017, 139, 15300–15303.
- 16L. Y. M. Eymann, A. G. Tskhovrebov, A. Sienkiewicz, J. L. Bila, I. Zikhovic, H. M. Ronnow, M. D. Wodrich, L. Vannay, C. Corminboeuf, P. Pattison, E. Solari, R. Scopelliti, K. Severin, J. Am. Chem. Soc. 2016, 138, 15126–15129.
- 17C. D. Martin, M. Soleilhavoup, G. Bertrand, Chem. Sci. 2013, 4, 3020–3030.
- 18O. Back, B. Donnadieu, P. Parameswaran, G. Frenking, G. Betrand, Nat. Chem. 2010, 2, 369–373.
- 19M. Y. Abraham, Y. Wang, Y. Xie, R. J. Gilliard, P. Wei, B. J. Vaccaro, M. K. Johnson, H. F. Schaefer, P. v. R. Schleyer, G. H. Robinson, J. Am. Chem. Soc. 2013, 135, 2486–2488.
- 20S. Kundu, S. Sinhababu, V. Chandrasekhar, H. W. Roesky, Chem. Sci. 2019, 10, 4727–4741.
- 21A. V. Zhukhovitskiy, J. Geng, J. A. Johnson, Chem. Eur. J. 2015, 21, 5685—5688.
- 22K. M. Stirk, L. K. M. Kiminkien, H. I. Kenttämaa, Chem. Rev. 1992, 92, 1649–1665.
- 23L. M. K. Kiminkinen, K. G. Stirk, H. I. Kenttamaa, J. Am. Chem. Soc. 1992, 114, 2027–2031.
- 24L. A. B. Moraes, M. N. Eberlin, J. Am. Chem. Soc. 1998, 120, 11136–11143.
- 25S. A. Morris, J. Wang, N. Zheng, Acc. Chem. Res. 2016, 49, 1957–1968.
- 26S. Maity, M. Zhu, R. S. Shinaberry, N. Zheng, Angew. Chem. Int. Ed. 2012, 51, 222–226; Angew. Chem. 2012, 124, 226–230.
- 27G. Gryn'ova, D. L. Marshall, S. J. Blanksby, M. L. Coote, Nat. Chem. 2013, 5, 474–481.
- 28A. Baishya, K. Kumar, M. K. Barman, H. S. Biswal, Inorg. Chem. 2017, 56, 9535–9546.
- 29D. Sánchez-Roa, T. G. Santiago, M. Fernández-Millán, T. Cuenca, P. Palma, J. Cámpora, M. E. G. Mosquera, Chem. Commun. 2018, 54, 12586–12589.
- 30N. M. Gallagher, A. V. Zhukhovitskiy, H. V.-T. Nguyen, J. A. Johnson, Macromolecules 2018, 51, 3006–3016.
- 31N. G. Connelly, W. E. Geiger, Chem. Rev. 1996, 96, 877–910.
- 32S. Stoll, A. Schweiger, J. Magn. Reson. 2006, 178, 42–55.
- 33A. D. Becke, J. Chem. Phys. 1988, 88, 2547–2553.
- 34Z. Li, S. Li, S. Liu, K. Huang, D. Fang, F. Wang, S. Peng, Electrochem. Solid-State Lett. 2011, 14, A 171–A173.